Chromium-6 in Water
http://patch.com/new-jersey/westfield/least-138-n-j-towns-have-drinking-water-toxin-made-famous-erin-brockovich
Well this isn't good. We drink bottled water, but use tap water to cook.
http://www.awwa.org/Portals/0/files/legreg/documents/UpdatedChromiumInDrinkingWaterSummaryFinal.pdf
The paper is from 2013, so there are likely updates. There's a comment about reporting requirements though, should levels exceed the standard water system need to notify their customers (doubtful that has changed):
"What are the safe levels of hexavalent chromium in drinking water?
The standard (MCL) for total chromium was set in 1992 and is based on allergic dermatitis (skin reactions). The MCL was based on the protective level indicated by the science at the time. The current federal maximum contaminant level for total chromium is 0.1 mg/L.
EPA is now reviewing data from a 2008 National Toxicology Program long-term animal study and other available research, which suggests that hexavalent chromium may be a human carcinogen if ingested. When the review is complete, EPA will consider this and other information to determine whether the drinking water standard for total chromium is sufficiently protective or whether it needs to be updated.
Regulatory Information
What is the current drinking water regulatory standard for chromium?
The current federal maximum contaminant level (MCL) for total chromium is 0.1 mg/L. The current California MCL for total chromium is set at 0.05 mg/L. There are currently no federal standards for hexavalent chromium – it is covered under the total chromium MCL.
If routine monitoring indicates that total chromium levels are above the MCL, water systems must take steps to reduce the total chromium below that level. Also, water systems must notify their customers as soon as practical, but no later than 30 days after the system learns of a violation (USEPA 2011)."
The best thing to do as JIT's starting in the right direction, is to get educated. What are our current levels, what are the standards, what should the standards be?
The original article is suggesting the regulations should be overhauled to be lower. Not just a little bit lower, and not just going down to a new lower standard, but setting the standard by the most rigid state (CA) ultimate goal. The current federal chromium standard as pointed out is 100 PPB, and California's is 50 PPB. Those are the numbers in JIT's paper - expressed in mg/L. The original article states that envronmental group wants to go to a standard of .02 PPB, not just 0.02 mg/L which is what the JIT article is in. The same thing in mg/L would be .00002 mg/L. That's 2500 times less that CA's standard, not 2.5 times. Your bottled spring water isn't going to pass that, likely only bottled distilled water will.
To compare what the current local numbers are, the tests for all types of chromium for both Washington Tsp and Hackettstown MUA are very similar, topping at 2.6 or 2.7 PPB respectively. I didn't see where the specific chromium-6 levels were tested for HMUA or WTMUA, but the Morris County MUA tested a couple of years ago and none of their MUA's went over 3 PPB but none went under 1.6 PPB. That means all of Morris County would fail a new standard if set at 0.02 PPB. And at that they would be failing by 80 times over the new standard.
If you are concerned about water quality, one of the best alternatives is to install a Reverse Osmosis system for your potable water use, which includes drinking and cooking. Reverse Osmosis systems will remove 99% of many impurities in water including chromium-6. A good five stage Reverse Osmosis system can be purchased for under $200.00. Installation is easy and can be accomplished by the homeowner if you are handy or a plumber. For additional information, please check the Premier web site:
https://www.premierh2o.com/
After smelling chlorine (or whatever HMUA is using) in the tap water for weeks recently, and now reading this article, I may be putting in a reverse osmosis system. And WHY do I pay for water, if it has potentially harmful levels of bad agents in it? (and please forgive me if I DON"T TRUST HMUA; it's in their best interests to say "the water is safe"... after all, Flint, MI has said "the water is safe"...)
Yes RO would remove all 3 types of Chromium and I would recommend to everyone...for unbiased info on any contaminant in the water I would suggest www.wqa.org
We really need to follow up on this. 2 of my cats had different cancers and drank tap water as well as a family member.
And the companies responsible for this toxin are probably long gone enjoying the good life and pristine waters.
BC-
You say- " Reverse Osmosis systems will remove 99% of many impurities in water including chromium-6."
But the remaining 1% wouldn't matter much if it were arsenic.
The levels they are talking about in the articles mentioned above are so minuscule that it is hard to comprehend, yet still harmful.
Is there such a thing as non-harmful water? Meaning even if we could go back in time and test water 300, 400, 1,000+ years ago, would it still have stuff in it that would be technically bad for us? (like radon from the ground itself- not something we caused, just how it is?)....
It is difficult to worry about stuff like this as we are all *constantly* exposed to harmful stuff no matter what we do. Plastic bottles bad, asbestos bad, exhaust gases bad, etc, etc.. but all around us is packaging, food additives, preservatives, electromagnetic waves, solar radiation, bananas, you name it... nobody gets out alive.
Josh, you are correct in your comments concerning the multitude of potentially harmful elements to which we are exposed on a daily basis and there is no way to totally eliminate that exposure. What matters though is that we endeavor to reduce the amount of exposure we encounter because it is often the cumulative effects or the way that different elements react with one another that are harmful. For instance, Arsenic and Radon in drinking water, even at low levels considered “safe” can build up in one’s system through long term exposure increasing the risk of cancer. One way to reduce one’s long term exposure to potentially harmful elements in our drinking water is through the use of a Reverse Osmosis system.
BC - Does a reverse osmosis system remove toxic impurities such as those recently thrown into our water by the water department's construction on Bald Eagle Road, and their "drainage", resulting in that "rotten eggs" smell, which has persisted in our hot water heaters? Love technical solutions...if they work.
Incorrect DannyC, hydrogen sulfide is smelly but not toxic.
At 99% be careful of what the exact levels removed of the exact contaminants you're interested in. If that original article's environmental group determines the standard, that level of reduction may or may not be enough. That's how strict it is.
PS - Arsenic as a heavy metal exactly builds up. But Radon is inert.
GC - So how does one get rid of the H2S smell? Cannot believe that it is not harmful to health. Certainly don't like smelling like it after taking a shower.
GC, you are correct that Arsenic is a heavy metal which can accumulate in one’s system. However, Radon is a radioactive gas that is far from inert. Although it does not accumulate in the body, long term exposure to Radon over time can lead to cancer. Inhalation can lead to lung cancer and ingestion can lead to gastrointestinal cancers. Also, Hydrogen Sulfide gas is a poison. When dissolved in water, Hydrogen Sulfide becomes a weak acid, which can be removed through chlorination of the water supply.
DannyC, if Sulfate got into your water it will produce Hydrogen Sulfide gas through the interaction of heat and the Magnesium anode in the water heater.
BC - Thanks for your expertise. But what specifically can I as a homeowner do to eliminate Hydrogen Sulfide?
Like arsenic, chromium-6 is also naturally occurring in nature, so just because it exists in the water supply doesn't necessarily mean it was put there by a nefarious business...
justintime, I don’t believe anyone is saying the Chromium-6 in the water was from nefarious sources. It is true that Chromium-6, also known as Hexavalent Chromium occurs naturally in the earth. However, it likely some degree of Chromate contamination of the groundwater we now draw upon occurred as a result of the leather tanning industry that once flourished here in Hackettstown. Remnants of the leather tanning industry can still be seen on Grand Avenue across from the baseball field. At that time, sanitary waste disposal practices were not followed as they are today and much of the waste was simply dumped into streams, rivers and the ground.
Hey guys - Still waiting for some practical advice on getting rid of H2S. I am sure that I am not the only homeowner in the area who is looking for this info.

I hope this is accurate - found on an interactive website: http://www.ewg.org/interactive-maps/2016-chromium6-lower-48.php
Allamuchy according to the chart:
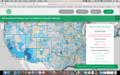
Here's a link to an interactive map of the nation, attached is a pic of all the Warren County water sources results:
http://www.ewg.org/interactive-maps/2016-chromium6-lower-48.php
BC - Radon is a 'noble' gas, by it's very definition it's inert. Don't confuse the term "inert" with "safe". Radon is not safe but it's still not something that builds up as you said. You can't change the laws of chemistry.
DannyC - H2S is a funny molecule with a dipole like water. The L shape sticks to stuff which means the water is all fine but the H2S lingers. It will not dissolve in water because it's so similar which means you can't just wash things. Most likely airing out what you can is the best idea. It eventually just dissipates.
I would have my water tested personally. That way you will know for sure what your dealing with.
I live in Great Meadows and have to worry about the Arial Lighting contamination from Asbury Rd. with my well. I live over a mile away from the place but the underground water supply travels past me. Every couple of years I have it tested for all those bad things. It's worth the $200-$300 that I've paid each time over the years. Maybe if a bunch of you get together to do this the company will give you a price break.
Good luck.
Magpie - If you have well water, it's a whole different ball game. It really has to be tested individually, and regularly. You also figure that no matter what the results, it's likely prudent to pay for some kind of filtration system. (and I'm not one of the filtration system dealers here)
BC
Hexavalent Chromium is a chemical based process and man made
Danny C oxidation is the best way to remove hydrogen sulfide from the water other than heating up the water to kill the bacteria and chlorination has nothing to do with it....might be new here but my profession is water and once again encourage everyone to visit WQA for fact based knowledge
GC, in response to your last post, perhaps you didn't read my statement concerning Radon. In case you missed it here it is again: GC, you are correct that Arsenic is a heavy metal which can accumulate in one’s system. However, Radon is a radioactive gas that is far from inert. Although it does not accumulate in the body, long term exposure to Radon over time can lead to cancer.
Radon is a radioactive gas. Being radioactive means it is reactive and therefore not inert as you stated.
Well I purchased a zero water filter last night.... removes chromium...lead..etc etc......so going to give my Brita a rest for a while and see how we like this filter.......
BC - "radioactive means it is reactive"
That is incorrect. Reactive means it combines with other molecules, it takes more than one to happen. Radioactivity is spontaneous without any other substance present because of atomic instability.
Don't confuse radioactivity with reactivity. The definition of inert is unreactive. The word "noble" when used with gases like Radon is a synonym of inert. Because of their chemistry they cannot react with other substances. They can however be radioactive but that is something different. Radon in particular is inert (reactivity) but also unstable (radioactivity).
BC and GC - So I guess it's cold water shower rinses until the H2S "dissipates". I was hoping for a solution like cleaning out and disinfecting the hot water tank to get rid of the problem sooner and for good.
GC, once again you seem to miss the point of this discussion, which centers on contaminants in drinking water. Keeping on that subject and realizing Radon is radioactive, the point is that it is not inert when ingested or inhaled. Radon, being radioactive, impacts the body’s cells by damaging DNA, which can lead to the development of cancer. The damage to DNA by Radon exposure is a reaction and is far from being inert.
For anyone else wanting to get rid of H2S from their hot water heater (*Hydrogen Sulfide gas is a poison" according to BC) :
http://www.affordablewater.us/Hydrogen-Sulfide-in-Water-How-To-Remove.aspx
so...why don't water plants like the HMUA have reverse osmosis filtering systems? Is this a silly question to ask? Why is that responsibility put upon the homeowner to get these filtering systems? Why can't the water plants install industrial ones, and give us safe water to begin with? Here is another silly question-When lots of us get our morning coffees, do these businesses have reverse osmosis filtering systems in their coffee-making stations? As soon as I read this thread, I thought of all the coffee we drink throughout the day!
coffeedrinker - Use only cold water filtered through a Zero Water filter and a Keurig coffee maker. Fill your big thermos with your favorite brewed coffee for the day and you should be good to go. Stay away from hot water until your hot water tank has been disinfected for H2S. Better off knowing what water and coffee are in what you drink, rather than relying on what commercial enterprises and HMUA put in it (or don't take out).
I live in Old Farm Village.... has anyone else noticed their water smelling very strongly of chlorine?!? I can't even drink it it so strong!
Chaz- call the water commission - they'll probably send someone out to take a sample....mine never smells but sometimes has that taste- but my refrigerator has a pretty good filter...
So is the water not safe for cooking?
Has anyone bought the Berkey water filter? It's pricey but I'm thinking about getting one.
For anyone who might have been “on the fence” concerning the potential lethal effects of Hydrogen Sulfide gas, please check out the story of a mother and daughter who died in Florida.
https://www.yahoo.com/news/m/ecd5a324-671c-3d7c-ab0a-e2f044bd72a0/ss_hydrogen-sulfide-inhalation.html
What's the LD 50 of H2S? What's vapor pressure? What's the water solubility and typical amount in our water? What's the volume of the resultant amount of water needed? What concentration of H2S would that have to be to be dangerous?
What FUD.
GC, at least it's for the purpose of trying to sell reverse osmosis filtration systems and not just to panic everyone for no reason (-;
Thanks for that, ianimal. Are we putting our engineering degrees to go use?? The thought never crossed my mind.
https://www.youtube.com/watch?v=PmQLoFfmqdY
Ianimal, my post was not for the purpose of selling RO units as your facetious comments suggest. Rather the information was offered for folks to research the technology and decide for themselves if they wanted to pursue.
GC, you can downplay the situation all you want. However, you must realize that not everyone shares your opinions. Folks with concerns should be encouraged to research all avenues before making decisions.
Really? Then why would you post an article about a Hydrogen Sulfide poisoning that had nothing to do with H2S contamination in a water supply in a drinking water quality thread? And include the ominous phrase "potentially lethal effects", after you had already plugged a reverse osmosis system three or four times? If you aren't a vendor trying to drum up business, you sure do a good job of playing one on the internet.
BC - That was not an opinion. I was asking to do a scientific analysis of what it would take to gather the enormous amount of water required to produce a dangerous H2S level in the air. All you have to do is plug the numbers into the formulas. How is that different from research?
ianimal, my comment concerning Hydrogen Sulfide (H2s) was pertinent and offered following comments from other posters where H2s was in their water supply as noted by the odor. Also, some posters had suggested H2s was not harmful, which is true in small amounts. However, H2s is lethal at higher amounts as the link to the article I provided indicates. Awareness is key here. I doubt the mother and daughter that succumbed to H2s poisoning on a Florida highway ever thought there was such a risk from their automobile battery. That’s why it’s best to err on the side of caution when it comes to H2s exposure.
GC, my comment concerning your “opinion” had to do with your response to ianimal; “at least it's for the purpose of trying to sell reverse osmosis filtration systems” where you were clearly stating your opinion.
The effects depend on how much hydrogen sulfide you breathe and for how long. Exposure to low levels over a prolonged period can have adverse health effects. Exposure to high concentrations even for a short duration can quickly lead to death. The amount of H2s can be measured by using a gas detection meter.
Concentration of H2s in air (parts per million (PPM):
0.00011-0.00033= Typical background concentrations
0.01-1.5= Odor threshold (when rotten egg smell is first noticeable to some). Odor becomes more offensive at higher levels.
2-5= Prolonged exposure may cause nausea, tearing of the eyes, headaches or loss of sleep. Airway problems (bronchial constriction) in some asthma patients.
100= Coughing, eye irritation, loss of smell after 2-15 minutes (olfactory fatigue). Altered breathing, drowsiness after 15-30 minutes. Throat irritation after 1 hour. Gradual increase in severity of symptoms over several hours. Death may occur after 48 hours.
Industry standards for H2s exposure is 10-20 PPM.
The afore mentioned information is readily available on line.
The concentration of H2s in water would dictate how much water would be needed to reach levels of concern.
BC, your comment related to H2S and comparing it to the situation on the Florida highway was akin to telling people that they should be frightened of chlorine in their drinking water because chlorine gas was used as a chemical weapon in WWI.
GC, problem is you want a definitive answer, which is not practical as it would depend on many factors. You seem to be a fairly intelligent individual. How about you educate the rest of us by developing a graph of various scenarios and share it with us. Also, the expose level could be more critical to some rather than others. Maybe you are a healthy individual and could withstand higher amounts of H2s exposure. However, the same would not necessarily be true for persons with health issues.
ianimal, your statement comparing my comment on H2s to the use of Chlorine gas as a weapon during WW-1 is completely without merit. You seem to have completely missed the point of my post, which centered on the potential lethal effects of H2s exposure and how it can occur from unlikely sources.
BC - No, you're not getting it again. It doesn't take a graph. You already stated what happens at various concentrations. Start with something dangerous like 10 PPM *in air*, use the VP and figure out what that is in *water*. Then compare that to the solubility.
GC, not sure why you are stuck on the amount of H2s in water when it is the concentration in the air that we breath that is a concern. It is important to identify potential sources of H2s whether it be from water or any other location for the purpose of mitigating exposure.
BC - "it is the concentration in the air that we breath that is a concern" That's the whole point and why I'm not stuck on it at all. I'm saying all along that H2S may be stinking and disgusting, but when you do the science the amount that could come from water is not a health concern.
GC, you state the amount of H2s from water is not a concern. However, that thinking totally overlooks people that might have health conditions where it would be concerning. Look, pollen in the air is nothing more than annoying to most of us. However, to those with asthma, that same amount of pollen can be life threatening so one needs to be aware and protect themselves. Same is true for peanuts. Most of us can consume peanuts and peanut products with no problems. However, for those with allergies, consumption of even a small amount can have an effect on their health or even be deadly. What I am trying to say and you seem to be missing is the awareness that H2s can have ill health effects even at low levels for those with certain health issues. Maybe you don’t fall into that category as your opinions seem to indicate but the awareness you lack is that others might and that matters.
Commenting is no longer available.